Triadimenol
Agent Name
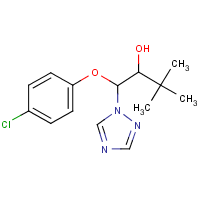
Triadimenol
CAS Number
55219-65-3
Formula
C14-H18-Cl-N3-O2
Major Category
Pesticides
Synonyms
(1RS,2RS,1RS,2SR)-1-(4-Chlorophenoxy)-3,3-dimethyl-1-(1H-1,2,4-triazol-1-yl)butan-2-ol (IUPAC); 1-(4-Chlorophenoxy)-3,3-dimethyl-1-(1H-1,2,4-triazol-1-yl)butan-2-ol; 2-(4-Chlorophenoxy)-1-tert-butyl-2-(1H-1,2,4-triazole-1-yl)ethanol; Bay KWG 0519; Bayfidan; Bayfidan EW; Baytan; Baytan 15; Baytan TF 3479B; KWG 0519; Spinnaker; Summit; Triafol; Triaphol; Tridan Fungicide; UK 199; beta-(4-Chlorophenoxy)-alpha-(1,1-dimethylethyl)-1H-1,2,4-triazole-1-ethanol; 1H-1,2,4-Triazole-1-ethanol, beta-(4-chlorophenoxy)-alpha-(1,1-dimethylethyl)-; Ethanol, 2-(4-chlorophenoxy)-1-tert-butyl-2-(1H-1,2,4-triazole-1-yl)-; alpha-tert-Butyl-beta-(4-chlorophenoxy)-1H-1,2,4-triazole-1-ethanol; [ChemIDplus]
Category
Fungicides
Description
Solid with a mild non-specific odor; [Merck Index] Formulated as emulsifiable, soluble, and flowable concentrates and wettable powder; [Reference #1]
Sources/Uses
Used as an agricultural fungicide and seed protectant; [Merck Index] The major metabolite of the triazole fungicide triadimefon; A broad spectrum systemic fungicide used as seed treatment for barley, corn, cotton, oats, rye, sorghum, and wheat; An import tolerance is also set for bananas; [Reference #1] Used as fungicide for table and wine grapes, cereals, and cereal seed treatment in the EU; [EFSA]
Comments
Commercial material is mixture of two diastereoisomers; [Merck Index] Increased liver enzyme activity (ALT and AST) and histopathological liver changes observed in 13-week feeding study of rats at the three highest doses tested (500, 1,500, and 4,500 ppm); A 15-day dermal study of rabbits produced a NOAEL greater than highest tested dose (250 mg/kg/day); Teratogenic effects seen in tadpoles (branchial arches) and cultured rat embryos (cranial nerve and ganglia); No evidence of mutagenicity; [HSDB] Not irritating to skin or eyes of rabbits; Not sensitizing to guinea pig skin; No adverse effects to reproductive performance observed in 2-generation study of rats; No evidence of primary embryotoxic or teratogenic effects in rats or rabbits administered by gavage in the most sensitive stage of gestation; No evidence of carcinogenicity in rats or mice; [IUCLID] Repeated dose studies have shown the liver to be the target organ of toxicity (enlargement with corresponding changes in clinical chemistry); Chronic studies of rats and mice also demonstrated hepatotoxicity; Multigenerational studies produced reduced pup survival during lactation and decreased fertility index in all generations in one study; Acute triadimefon (structurally and metabolically similar fungicide) neurotoxicity studies of rats showed reversible hyperactivity and increased motor and locomotor activity without neuropathological changes; Sub-chronic studies produced consistent effects with no increase in severity observed; Does not possess potential for mutagenicity or carcinogenicity; [EFSA] See "Triadimefon."
Reference Link #1
Biomedical References
Exposure Assessment
Vapor Pressure
3.1E-10 mm Hg
Lethal Concentration
LC50 (rat) = 2,580 mg/m3
Adverse Effects
Hepatotoxin
Hepatoxic (a) from occupational exposure (secondary effect) or (b) in animal studies or in humans after ingestion
Diseases, Processes, and Activities Linked to This Agent
Processes
Industrial Processes with risk of exposure: